Formic Acid
Formic acid, with the chemical formula HCOOCH CH2 H2O, is a compound that might not be on everyone’s radar but plays an essential role in various industries and applications. Often overshadowed by its more famous counterparts like acetic acid, formic acid deserves some recognition for its unique properties and versatility. From agriculture to food preservation, this simple molecule boasts a range of uses that are vital for everyday life.
But what exactly is formic acid? How does its structure contribute to its diverse functionalities? And why should we care about it? As we dive into the world of formic acid, you’ll discover fascinating insights into its chemistry and explore how it’s shaping sustainable practices in our modern world. Buckle up as we embark on this enlightening journey through the realm of HCOOCH CH2 H2O!
Chemical Properties and Structure
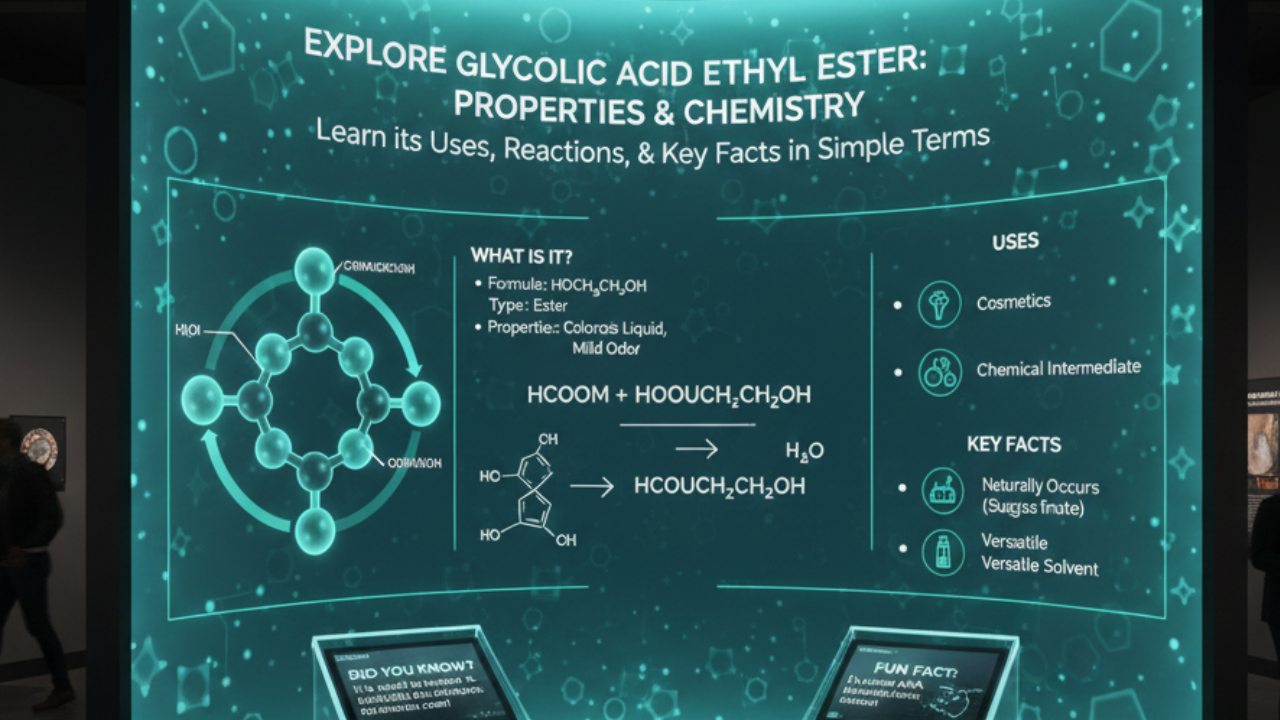
Formic acid, with the chemical formula HCOOH, is a simple carboxylic acid. Its structure features a single carbon atom bonded to both a hydroxyl group (-OH) and a carbonyl group (C=O). This unique arrangement gives rise to its distinct properties.
The molecule is polar due to the presence of the electronegative oxygen atoms. This polarity contributes to its high solubility in water, making it an effective solvent for various applications.
Formic acid has a relatively low boiling point of 100.8°C (213.4°F), which makes it volatile under standard conditions. Additionally, it exhibits strong acidic behavior with a pKa value around 3.75, allowing it to donate protons easily in solution.
It’s also worth noting that formic acid can act as both an oxidizing and reducing agent under certain conditions. These versatile characteristics make it fascinating from both scientific and practical perspectives.
Uses of Formic Acid in Various Industries
Formic acid serves as a versatile compound across several industries. In agriculture, it acts as an effective preservative in silage and improves feed quality for livestock. Farmers appreciate its ability to inhibit harmful bacteria while promoting beneficial microorganisms.
The textile industry also finds value in formic acid. It is used during the dyeing process to help achieve vibrant colors and enhance fabric properties. This chemical helps maintain pH levels during production, ensuring consistent results.
In pharmaceuticals, formic acid plays a role in drug formulation and synthesis of active ingredients. Its unique properties allow for precise adjustments in various chemical reactions.
Additionally, the energy sector explores formic acid’s potential as an eco-friendly fuel alternative. Researchers are investigating its use in hydrogen storage systems due to its high energy density and lower environmental impact compared to traditional fuels.
Benefits and Risks of Using Formic Acid
Formic acid, or HCOOCH CH2 H2O, offers notable benefits across various sectors. In agriculture, it serves as a potent preservative for silage and animal feed. Its effectiveness in controlling pests makes it invaluable in the farming industry.
However, using formic acid isn’t without risks. It’s corrosive and can cause skin burns or respiratory issues upon exposure. Proper handling is crucial to ensure safety.
In industrial applications, its role as a reducing agent is well recognized. It helps produce chemicals like methanol efficiently. Yet, the potential hazards of improper storage must be considered seriously.
Environmental implications are also significant. While biodegradable in nature, excessive use can lead to soil and water contamination if not managed appropriately. Balancing its advantages with these risks requires diligence from all users.
How to Handle Formic Acid Safely
When handling formic acid, safety should be your top priority. Always wear appropriate personal protective equipment (PPE), including gloves, goggles, and lab coats. This prevents direct contact with the skin and eyes.
Ensure that you work in a well-ventilated area or use a fume hood. Formic acid can release harmful vapors that may irritate your respiratory system. Good ventilation helps mitigate this risk.
Be familiar with the material safety data sheet (MSDS) for formic acid before beginning any work. Understanding its hazards is crucial for safe handling.
Store formic acid in a cool, dry place away from incompatible substances like strong oxidizers or bases. Use designated containers labeled clearly to avoid confusion.
In case of spills, act quickly but cautiously. Neutralize small spills with sodium bicarbonate before cleaning up thoroughly to minimize risks associated with exposure.
Alternative Uses of Formic Acid in Sustainable Practices
Formic acid, with its chemical formula HCOOCH CH2 H2O, is more than just a simple organic compound. It has found innovative applications in sustainable practices that benefit both the environment and industries.
One notable use is as a natural pesticide. Its ability to disrupt pests without harming beneficial insects makes it an eco-friendly alternative for agriculture. Farmers can protect crops while minimizing chemical runoff into soil and water systems.
Another exciting application lies in energy production. Researchers explore formic acid as a potential fuel source due to its high energy density. This could lead to cleaner alternatives for powering vehicles and reducing greenhouse gas emissions.
Moreover, formic acid plays a role in carbon capture technologies. By converting captured CO2 into useful products, it contributes to efforts aimed at combating climate change while promoting resource efficiency across various sectors.
Conclusion:
The future of formic acid, represented by the formula HCOOCH CH2 H2O, is bright as researchers explore its vast potential in various fields. This versatile compound has already made a significant impact in industries like agriculture, textiles, and pharmaceuticals. Its role as a preservative and antibacterial agent continues to gain attention.
Sustainability is becoming increasingly important for businesses worldwide. Formic acid shows promise here too. It can be used in renewable energy systems and biofuels, contributing to greener alternatives that reduce our reliance on fossil fuels.
Moreover, advancements in chemistry may lead to new applications we haven’t even considered yet. The ongoing exploration of formic acid’s properties could unlock innovative solutions for pressing global challenges.
As more industries adopt sustainable practices, the demand for efficient chemicals like formic acid will likely rise. Staying informed about its uses and safety measures is essential for anyone involved with this compound.
With ongoing research and development efforts focused on maximizing its benefits while minimizing risks, formic acid stands at the crossroads of traditional chemistry and modern sustainability initiatives. As we move forward into an era where eco-friendly practices are prioritized, the relevance of HCOOCH CH2 H2O will only continue to grow.